产品检索


联系光柏™
张先生(销售经理)
手机:13724839064
电话:020-28940181
传真:020-34764684
电邮:miken@Goodpex.com

LED’s Colors and materials
更新时间:2010-07-22
LED’s Colors and materials
Ultraviolet and blue LEDs,White light,RGB systems,Phosphor-based LEDs,Other white LEDs,Organic light-emitting diodes (OLEDs),Quantum dot LEDs (experimental),
Conventional LEDs are made from a variety of inorganic semiconductor materials, the following table shows the available colors with wavelength range, voltage drop and material:
|
|
Color |
Wavelength (nm) |
Voltage (V) |
Semiconductor Material |
| Infrared | λ > 760 | ΔV < 1.9 | Gallium arsenide (GaAs) Aluminium gallium arsenide (AlGaAs) |
|
| Red | 610 < λ < 760 | 1.63 < ΔV < 2.03 | Aluminium gallium arsenide (AlGaAs) Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) |
|
| Orange | 590 < λ < 610 | 2.03 < ΔV < 2.10 | Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) |
|
| Yellow | 570 < λ < 590 | 2.10 < ΔV < 2.18 | Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) |
|
| Green | 500 < λ < 570 | 1.9[42] < ΔV < 4.0 | Indium gallium nitride (InGaN) / Gallium(III) nitride (GaN) Gallium(III) phosphide (GaP) Aluminium gallium indium phosphide (AlGaInP) Aluminium gallium phosphide (AlGaP) |
|
| Blue | 450 < λ < 500 | 2.48 < ΔV < 3.7 | Zinc selenide (ZnSe) Indium gallium nitride (InGaN) Silicon carbide (SiC) as substrate Silicon (Si) as substrate — (under development) |
|
| Violet | 400 < λ < 450 | 2.76 < ΔV < 4.0 | Indium gallium nitride (InGaN) | |
| Purple | multiple types | 2.48 < ΔV < 3.7 | Dual blue/red LEDs, blue with red phosphor, or white with purple plastic |
|
| Ultraviolet | λ < 400 | 3.1 < ΔV < 4.4 | Diamond (235 nm)[43] Boron nitride (215 nm)[44][45] Aluminium nitride (AlN) (210 nm)[46] Aluminium gallium nitride (AlGaN) Aluminium gallium indium nitride (AlGaInN) — (down to 210 nm)[47] |
|
| White | Broad spectrum | ΔV = 3.5 | Blue/UV diode with yellow phosphor |
Ultraviolet and blue LEDs
Blue LEDs.
 Blue LEDs are based on the wide band gap semiconductors GaN (gallium nitride) and InGaN (indium gallium nitride). They can be added to existing red and green LEDs to produce the impression of white light, though white LEDs today rarely use this principle.
Blue LEDs are based on the wide band gap semiconductors GaN (gallium nitride) and InGaN (indium gallium nitride). They can be added to existing red and green LEDs to produce the impression of white light, though white LEDs today rarely use this principle.
The first blue LEDs were made in 1971 by Jacques Pankove (inventor of the gallium nitride LED) at RCA Laboratories.[48] These devices had too little light output to be of much practical use. However, early blue LEDs found use in some low-light applications, such as the high-beam indicators for cars. In the late 1980s, key breakthroughs in GaN epitaxial growth and p-type doping ushered in the modern era of GaN-based optoelectronic devices. Building upon this foundation, in 1993 high brightness blue LEDs were demonstrated.
By the late 1990s, blue LEDs had become widely available. They have an active region consisting of one or more InGaN quantum wells sandwiched between thicker layers of GaN, called cladding layers. By varying the relative InN-GaN fraction in the InGaN quantum wells, the light emission can be varied from violet to amber. AlGaN aluminium gallium nitride of varying AlN fraction can be used to manufacture the cladding and quantum well layers for ultraviolet LEDs, but these devices have not yet reached the level of efficiency and technological maturity of the InGaN-GaN blue/green devices. If the active quantum well layers are GaN, as opposed to alloyed InGaN or AlGaN, the device will emit near-ultraviolet light with wavelengths around 350–370 nm. Green LEDs manufactured from the InGaN-GaN system are far more efficient and brighter than green LEDs produced with non-nitride material systems.
With nitrides containing aluminium, most often AlGaN and AlGaInN, even shorter wavelengths are achievable. Ultraviolet LEDs in a range of wavelengths are becoming available on the market. Near-UV emitters at wavelengths around 375–395 nm are already cheap and often encountered, for example, as black light lamp replacements for inspection of anti-counterfeiting UV watermarks in some documents and paper currencies. Shorter wavelength diodes, while substantially more expensive, are commercially available for wavelengths down to 247 nm. As the photosensitivity of microorganisms approximately matches the absorption spectrum of DNA, with a peak at about 260 nm, UV LED emitting at 250–270 nm are to be expected in prospective disinfection and sterilization devices. Recent research has shown that commercially available UVA LEDs (365 nm) are already effective disinfection and sterilization devices.
Deep-UV wavelengths were obtained in laboratories using aluminium nitride (210 nm), boron nitride (215 nm) and diamond (235 nm).
White light
There are two primary ways of producing high intensity white-light using LEDs. One is to use individual LEDs that emit three primary colors[54]—red, green, and blue—and then mix all the colors to produce white light. The other is to use a phosphor material to convert monochromatic light from a blue or UV LED to broad-spectrum white light, much in the same way a fluorescent light bulb works.
Due to metamerism, it is possible to have quite different spectra that appear white.
RGB systems
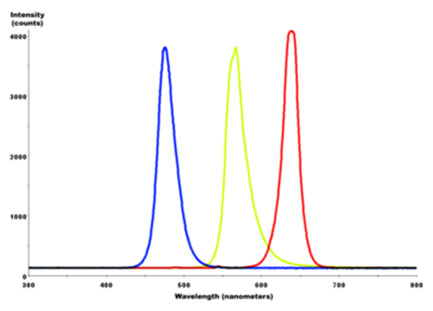 Combined spectral curves for blue, yellow-green, and high brightness red solid-state semiconductor LEDs. FWHM spectral bandwidth is approximately 24–27 nm for all three colors.
Combined spectral curves for blue, yellow-green, and high brightness red solid-state semiconductor LEDs. FWHM spectral bandwidth is approximately 24–27 nm for all three colors.
White light can be produced by mixing differently colored light, the most common method is to use red, green and blue (RGB). Hence the method is called multi-colored white LEDs (sometimes referred to as RGB LEDs). Because its mechanism is involved with electro-optical devices to control the blending and diffusion of different colors, this approach is little used to produce white lighting. Nevertheless this method is particularly interesting in many applications because of the flexibility of mixing different colors, and, in principle, this mechanism also has higher quantum efficiency in producing white light.
There are several types of multi-colored white LEDs: di-, tri-, and tetrachromatic white LEDs. Several key factors that play among these different approaches include color stability, color rendering capability, and luminous efficacy. Often higher efficiency will mean lower color rendering, presenting a trade off between the luminous efficiency and color rendering. For example, the dichromatic white LEDs have the best luminous efficacy (120 lm/W), but the lowest color rendering capability. Conversely, although tetrachromatic white LEDs have excellent color rendering capability, they often have poor luminous efficiency. Trichromatic white LEDs are in between, having both good luminous efficacy (>70 lm/W) and fair color rendering capability.
 What multi-color LEDs offer is not merely another solution of producing white light, but is a whole new technique of producing light of different colors. In principle, most perceivable colors can be produced by mixing different amounts of three primary colors, and this makes it possible to produce precise dynamic color control as well. As more effort is devoted to investigating this technique, multi-color LEDs should have profound influence on the fundamental method which we use to produce and control light color. However, before this type of LED can truly play a role on the market, several technical problems need to be solved. These certainly include that this type of LED's emission power decays exponentially with increasing temperature,resulting in a substantial change in color stability. Such problems are not acceptable for industrial usage. Therefore, many new package designs aimed at solving this problem have been proposed and their results are now being reproduced by researchers and scientists.
What multi-color LEDs offer is not merely another solution of producing white light, but is a whole new technique of producing light of different colors. In principle, most perceivable colors can be produced by mixing different amounts of three primary colors, and this makes it possible to produce precise dynamic color control as well. As more effort is devoted to investigating this technique, multi-color LEDs should have profound influence on the fundamental method which we use to produce and control light color. However, before this type of LED can truly play a role on the market, several technical problems need to be solved. These certainly include that this type of LED's emission power decays exponentially with increasing temperature,resulting in a substantial change in color stability. Such problems are not acceptable for industrial usage. Therefore, many new package designs aimed at solving this problem have been proposed and their results are now being reproduced by researchers and scientists.
Phosphor-based LEDs
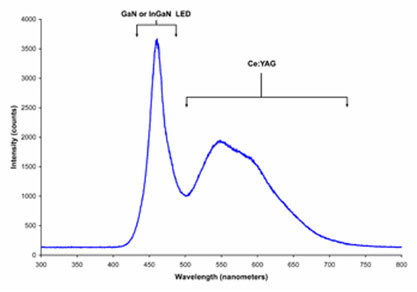
Spectrum of a “white” LED clearly showing blue light which is directly emitted by the GaN-based LED (peak at about 465 nm) and the more broadband Stokes-shifted light emitted by the Ce3+:YAG phosphor which emits at roughly 500–700 nm.
This method involves coating an LED of one color (mostly blue LED made of InGaN) with phosphor of different colors to produce white light, the resultant LEDs are called phosphor-based white LEDs.A fraction of the blue light undergoes the Stokes shift being transformed from shorter wavelengths to longer. Depending on the color of the original LED, phosphors of different colors can be employed. If several phosphor layers of distinct colors are applied, the emitted spectrum is broadened, effectively increasing the color rendering index (CRI) value of a given LED.
Phosphor based LEDs have a lower efficiency than normal LEDs due to the heat loss from the Stokes shift and also other phosphor-related degradation issues. However, the phosphor method is still the most popular technique for manufacturing high intensity white LEDs. The design and production of a light source or light fixture using a monochrome emitter with phosphor conversion is simpler and cheaper than a complex RGB system, and the majority of high intensity white LEDs presently on the market are manufactured using phosphor light conversion.
The greatest barrier to high efficiency is the seemingly unavoidable Stokes energy loss. However, much effort is being spent on optimizing these devices to higher light output and higher operation temperatures. For instance, the efficiency can be increased by adapting better package design or by using a more suitable type of phosphor. Philips Lumileds' patented conformal coating process addresses the issue of varying phosphor thickness, giving the white LEDs a more homogeneous white light. With development ongoing, the efficiency of phosphor based LEDs is generally increased with every new product announcement.
Technically the phosphor based white LEDs encapsulate InGaN blue LEDs inside of a phosphor coated epoxy. A common yellow phosphor material is cerium-doped yttrium aluminium garnet (Ce3+:YAG).
White LEDs can also be made by coating near ultraviolet (NUV) emitting LEDs with a mixture of high efficiency europium-based red and blue emitting phosphors plus green emitting copper and aluminium doped zinc sulfide (ZnS:Cu, Al). This is a method analogous to the way fluorescent lamps work. This method is less efficient than the blue LED with YAG:Ce phosphor, as the Stokes shift is larger and more energy is therefore converted to heat, but yields light with better spectral characteristics, which render color better. Due to the higher radiative output of the ultraviolet LEDs than of the blue ones, both approaches offer comparable brightness. Another concern is that UV light may leak from a malfunctioning light source and cause harm to human eyes or skin.
Other white LEDs
Another method used to produce experimental white light LEDs used no phosphors at all and was based on homoepitaxially grown zinc selenide (ZnSe) on a ZnSe substrate which simultaneously emitted blue light from its active region and yellow light from the substrate.
Organic light-emitting diodes (OLEDs)
Main article: Organic light-emitting diode If the emitting layer material of the LED is an organic compound, it is known as an organic light emitting diode (OLED). To function as a semiconductor, the organic emitting material must have conjugated pi bonds. The emitting material can be a small organic molecule in a crystalline phase, or a polymer. Polymer materials can be flexible; such LEDs are known as PLEDs or FLEDs.
Compared with regular LEDs, OLEDs are lighter, and polymer LEDs can have the added benefit of being flexible. Some possible future applications of OLEDs could be:
Inexpensive, flexible displays
Light sources
Wall decorations
Luminous cloth
OLEDs have been used to produce visual displays for portable electronic devices such as cellphones, digital cameras, and MP3 players. Larger displays have been demonstrated, but their life expectancy is still far too short (<1,000 hours) to be practical[citation needed].
Today, OLEDs operate at substantially lower efficiency than inorganic (crystalline) LEDs.
Quantum dot LEDs (experimental)
A new technique developed by Michael Bowers, a graduate student at Vanderbilt University in Nashville, involves coating a blue LED with quantum dots that glow white in response to the blue light from the LED. This technique produces a warm, yellowish-white light similar to that produced by incandescent bulbs.
Quantum dots are semiconductor nanocrystals that possess unique optical properties. Their emission color can be tuned from the visible throughout the infrared spectrum. This allows quantum dot LEDs to create almost any color on the CIE diagram. This provides more color options and better color rendering than white LEDs. Quantum dot LEDs are available in the same package types as traditional phosphor based LEDs.
In September 2009 Nanoco Group announced that it has signed a joint development agreement with a major Japanese electronics company under which it will design and develop quantum dots for use in light emitting diodes (LEDs) in liquid crystal display (LCD) televisions.
| 下一个: | LED Technology Physics Efficiency and operational parameters |
| 上一个: | Lamp Base Examples |